Protein Factory Reveals Its Secrets (Part 2)
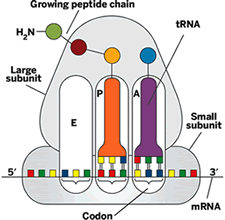 As peptide bond formation occurs, the mRNA and tRNAs translocate-that is, they shimmy over one codon length. The P-site tRNA moves to the E site, where it gets ready to leave the ribosome, and the A-site tRNA moves to the P site, opening a space on the A site for a new tRNA. When the ribosome reaches an mRNA codon that signals a stop, the protein chain is released for use by the cell. The protein makes its getaway from the ribosome through a tunnel in the large subunit.
As peptide bond formation occurs, the mRNA and tRNAs translocate-that is, they shimmy over one codon length. The P-site tRNA moves to the E site, where it gets ready to leave the ribosome, and the A-site tRNA moves to the P site, opening a space on the A site for a new tRNA. When the ribosome reaches an mRNA codon that signals a stop, the protein chain is released for use by the cell. The protein makes its getaway from the ribosome through a tunnel in the large subunit.The ribosome doesn't carry out protein translation all by itself. It gets assistance from cofactors like EF-Tu, which delivers tRNA-amino acid complexes to the ribosome; EF-G, which catalyzes translocation; and release factors, which help synthesized proteins to exit the ribosome.
Over the past few decades, researchers have worked toward a much more detailed understanding of the way the ribosome works on an atomic level. Efforts go back at least to the 1960s, when Masayasu Nomura, now professor of biological chemistry at the University of California, Irvine, and coworkers showed that the ribosome could assemble spontaneously from its component RNAs and proteins. In the 1970s and '80s, Harry Noller, director of the Center for Molecular Biology of RNA at UC Santa Cruz, and coworkers used chemical modification studies to identify key ribosomal nucleotides and obtained evidence for the idea that translocation occurs in two discrete yet coupled steps.
Structural studies have spearheaded much of the progress since then in understanding the ribosome. Crystals of a ribosome subunit for crystallographic investigation were first made in 1980 by the late biochemist H. G. Wittmann of Max Planck Institute for Molecular Genetics, Berlin; structural biologist Ada E. Yonath of Weizmann Institute, Rehovot, Israel; and coworkers. Such crystals were initially prone to radiation damage. But in 1986, Yonath's group showed that the analysis of flash-frozen crystals, a technique called cryo-crystallography, can minimize radiation damage to the ribosome. This technique has improved the quality of data from subsequent crystallography of the ribosome and other biomolecules.
In the 1990s, Howard Hughes Medical Institute Investigator Joachim Frank of both the Wadsworth Center, Albany, N.Y., and the State University of New York, Albany, and his coworkers developed single-particle cryo-electron microscopy (cryo-EM) and began using it to study ribosome structure. Single-particle cryo-EM is a technique for imaging sets of individual molecules embedded in a thin layer of ice. Cryo-EM can be used to observe a greater variety of functionally interesting forms of biomolecules than is possible with crystallography. But cryo-EM can't normally attain atomic resolution, whereas X-ray crystallography can, so Frank and his coworkers often use crystallographic data to refine their cryo-EM maps.
By applying this combined approach to ribosomes, "we have been able to visualize a plethora of different processes that weren't seen before," Frank says. For instance, he and his coworkers showed how tRNA, upon entering the ribosome with EF-Tu, acts like a molecular spring by distorting as it contacts mRNA and then straightening as it enters the A site. They also found that when EF-G binds to the ribosome to induce translocation, the small and large subunits make a ratchet motion by rotating about 10 degrees about each other.
In 2000, several teams of researchers captured atomic (approximately 3-Å resolution) or near-atomic crystal structures of ribosome subunits. A Yale University group led by Thomas A. Steitz and Peter B. Moore reported a 2.4-Å structure of the large subunit, which continues to be the highest resolution ribosome structure of any kind. This study helped confirm that the ribosome is a ribozyme by showing that RNA predominates in the active site. The group subsequently obtained additional structures of the large subunit bound to various substrates, among them antibiotics and transition-state analogs.


No comments:
Post a Comment